You are a brand or a manufacturer with a mission to develop an idea into a cosmetic product. It must be all singing and dancing for many markets across the world! How can this be achieved?
You need a team experienced in many areas for a smooth and productive result.
The formulation development of a cosmetic product is an art involving knowledge in different areas. Not only will raw materials need to be combined to create a formula, but the formula needs to follow a manufacturing process which will be achievable and scalable during the industrial manufacturing process, so times, temperatures, mixing stages need to be carefully calibrated. Furthermore, the finished product must meet the desired performance and efficacy claims to appeal the target consumers and it must be safe and compliant with all the chosen markets.
A cosmetic formulator will have to approach the development process considering the ever-evolving regulations governing the cosmetic field, knowing ingredients restrictions and their availability in the future, understanding the technology behind the ingredients to be able to meet the desired claims. Compliance must be considered as a first step and observed through the whole formulation development process.
Formulation development
The formulation development is a multi-steps process, where several factors are involved. Formulation is not the only aspect, but a coordination of this with marketing, with safety evaluation and with efficacy evaluation comes into play. In this way a formulator becomes a key figure to achieve a successful path to a product launch.
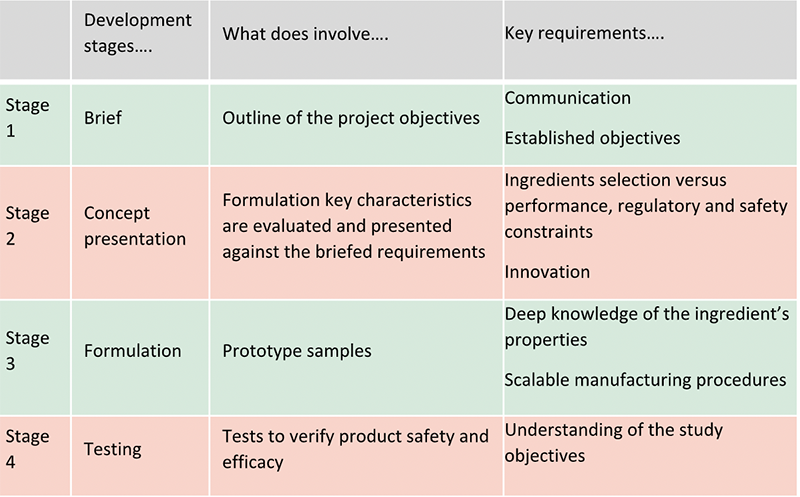
First step to consider is the brief. This is born from an idea and brainstorm process to capture the brand vision and outline the project objectives. A detailed brief is of utmost importance to avoid delays later. Communication between the formulator and the marketing must be open and clear with all the steps involved in the process being reviewed. Product format, characteristics (appearance, consistency etc), functions (cleaning, hydrating etc), accreditations (organic, natural), brand policies (sustainability, palm free, vegan, cruelty free), claims, target cost and testing required to support them, markets – all of them must be defined at the beginning. Deviations from established objects need be avoided to be able to stick to the timelines and budget resources.
Second step is the concept presentation from the formulator to the briefing team. This is a further refinement of the initial brief where key concepts and product characteristics are evaluated by the formulator against the agreed requirements. Which are the best key ingredients to meet the claims? Which is the required texture to meet the desired performance? The original abstract idea can start to turn into a reality. The formulator must respond to the need of creating a unique product. There must be balance between the formulating solutions and the required constraints (regulations, safety, etc). Ingredients must be selected carefully to make sure they meet not only the desired performance to appeal consumers and meet the claimed efficacy, but also to meet the regulatory and safety requirements. For example, some countries may restrict the use of an ingredient which is instead allowed in another country whereas some ingredients have better efficacy data to achieve the requested claims. Innovation is also a must to stand out from the crowd and to meet unique selling points of the required product. Therefore, even the most experienced formulator will need to keep updated with new developments. The concept presentation will allow the brand to be guided through the available possibilities to meet their vision in an ever-growing ingredients offering.
Once the concept presentation has been agreed, the formulation starts. This is where art and science come together. Formulation is complex and experience and continuous research and learning of ingredients is fundamental. This will require the deep knowledge of the ingredients properties and reviewing of the supplier’s data. Then the optimal method to combine ingredients will have to be developed to avoid incompatibilities in the formula’s stability, or unsuitability with final packaging and difficulties with scaling up the manufacturing procedures. Ingredients are combined to make prototypes to match the texture, feel and odour envisaged. Initial evaluation and further tweaks following the brand feedback can follow until a final agreement is met. This can involve a few options.
Once formulation prototype(s) has been agreed and approved by the brand, product will need to undergo several tests to verify its safety and efficacy. Tests can include stability, compatibility, preservative effectiveness test, claims substantiation studies. A good stability and compatibility plan will take a minimum of 12 weeks. Preservative effectiveness around 6 weeks. Claims support can take longer up to a couple of months. If any of them fail, the whole testing process will need repeating. Formulator’s experience can help to minimise the risks associated with this stage. Discussion and continuous collaboration with testing houses will help the formulator to understand the study objectives and apply the best suited efficacy and safety test to support claims.
A summary of the main stages of a formulation development process is outlined in below table:
Regulatory role
Creativity is complemented and optimied by a regulatory support. A regulatory advisor will not only work to make sure current regulations across different markets are applied but will also contribute to the creativity process to suggest changes aimed to improved product safety and to communicate correct claims to consumers.
The regulatory role will be vital from the start of the formulation development process; for example, in the evaluation of new ingredients or in the review of existing ones to propose alternatives with improved efficacy or better environmental profile.
Experience in the difference of regulations among countries and constant contacts with the industry will make the development process faster and efficient. A continuous knowledge renovation will help formulation work (for example avoiding ingredients likely to be banned or restricted) and avoid future issues in the market areas. This can also be useful for current ranges where proactive reformulation avoids unexpected legislation and consequential loss of sales.
When the formulation has been finalised by the brand, more stages need to be considered during a product development and the role of regulatory can help further.
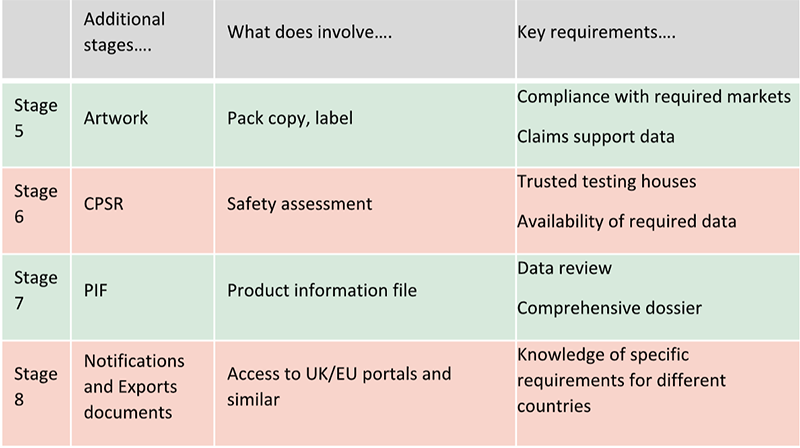
Artwork creation and review is one of these steps. This is when labels are to be finalised. Regulatory support is necessary to confirm compliance with the many market requirements, including claims wording to match support data.
A Cosmetic Product Safety Report (CPSR) is then required to confirm the finished product is safe for use. Collaboration of regulatory and creativity teams and trusted testing houses will ensure all the required data are available to proceed quicker to complete this stage.
All the information related to the finished product are now ready to be compiled in a Product Information File (PIF). Responsible persons are required to promptly provide this document if requested by authorities. Data review by regulatory and creativity teams will ensure all the required information are available to provide a comprehensive document. Additional steps can be required depending on markets requirements. For example, notifications of finished products before launch are required in Europe and UK. Safety Data Sheets and specific documents are required to export in different countries. Regulatory support can help brands and/or manufacturing companies to fulfil specific requirements for different countries (registration, etc).
All these additional stages can be summarised as per below table:
Consultancy services: an extra hand…
The success of a smooth development process is strongly dependant on an active and effective communication between marketing, creativity and regulatory. These departments can be present in a large manufacturer company but often it is difficult to find the right level of communication and experience to efficiently achieve the desired results.
Consultancy services can help to overcome this. Experience, extensive and continuous contacts in the industry and not least passion will make the consultancy services a very helpful tool to brands and manufacturers to offer innovation and efficient solutions. The Cosmetic Experts can cover a wide range of formulation development, whether it involves novel ideas to add to your current range or replicate your desired benchmarks or trouble shooting your existing formulations.
The aim is to deliver your vision while addressing all the regulatory requirements and comply with the different markets.
Please visit their website to learn more - www.thecosmeticexperts.co.uk
